























R&D
We contribute to promoting human health.
We are the global leader in stem cell therapies




What is GMP Center? Good manufacturing practices (GMP) are quality control standards required by licensed agencies for the manufacture and sale of food, pharmaceuticals, cosmetics and medical devices, etc. It provides the minimum standards necessary to maintain a consistent quality level in manufacturing. The company has facilities suitable for GMP and is capable of cultivating 6000 lots per year.

GMP Center History
Possesses original technology of separate culture of fat stem cells Established more than 100 cooperative hospitals in Korea to promote customer convenience Stem cell global treatment available at over 50 partner hospitals overseas









October 2015
Completion of 1 GMP Center
May 2016
Ministry of Food and Drug
Safety GMP Survey

April 2019
Completion of 2 GMP Center
Can culture 6000Lot
cells per year
(500Lot per month)

Management system
based on GMP facility of the Ministry of Food and
Drug Safety

Atopic dermatitis
stem cell treatment clinical approval
(clinical phase 2 in progress)

Acquired CPC(Cell Processing Center)
permission from the Ministry of Health,
Labor and Welfare of Japan

Acquired the 1st human cell etc
management business in Korea

Acquired CPC(Cell Processing Center)
for the 1st time in Korea



Clean Room
Airborne particulates are managed below the specified cleanliness
Maintain cleanliness to protect raw materials used in manufacturing space
Area or space designed to manage the environment such as temperature and humidity
Built-in incubator planned from the design stage



Biological Safety Cabinet (BSC)
Used in laboratories dealing with infectious substances such as pathogens
Basic safety equipment used to protect handling materials, experimenter's safety, and environment
It is classified into class1, class2, and class3 according to the type and speed of inflow and exhaust air in the equipment



CO2 incubator
Tissue culture thermostat equipped with the function of maintaining the gaseous environment with carbon dioxide added
Device for adjusting the pH of the culture medium
Supply of set CO2 and maintenance of temperature



Centrifuge
Purpose of dividing the homogenate into several parts using centrifugal force
Spins at high speed and separates materials according to particle size and density



Inverted Microscope
Capture the image with an objective lens and refract it with a prism to observe it from the front eyepiece
The morphology and function of the proliferating cultured cells can be observed



Liquid nitrogen tank
It is used for long-term preservation of cells at cryogenic conditions.
It is liquefied nitrogen and exists as a liquid at -196 ° C under atmospheric pressure.



Air Handling Unit
A device that supplies outside air into the clean room
Heating coil, cooling coil, humidifier, air filter, blower unit
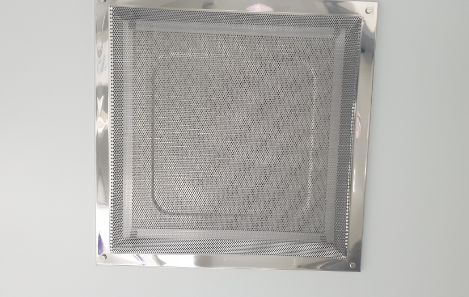

HEPA Filter
HEPA: High Efficiency Particulate Air (99.97%)
Air purification device developed to purify airborne particulates
For commercial use that requires an entirely clean air environment for safety